Synthesis and Application of several tertiary amines for preparing hydrophilic block silicone oil
Liu Ruiyun
OSiC Performance Materials
Textile Auxiliaries
No.1218, Songsheng Road
201600 Shanghai
China
Abstract: Four kinds of hydrophilic block silicone oil were obtained by reaction of tetramethylethylenediamine, tetramethyl propylenediamine, tetramethylhexanediamine and bis ( dimethylaminoethyl ) ether with epoxy silicone oil. The physicochemical properties of these four hydrophilic softeners, such as acid resistance, alkali resistance, anion resistance and shear resistance, were investigated and their application properties were studied. The results show that the hydrophilic block silicone oil synthesized by using three tertiary amines of tetramethylpropylenediamine, tetramethylhexamethylenediamine and bis(dimethylaminoethyl)ether has the same handle and hydrophilic properties.
Key words: Tertiary amine; hydrophilic; block silicone oil; properties
1. Introduction
Textiles treated with conventional silicone softeners generally suffer from poor hydrophilicity, hot stuffy wearing, and reduced comfort. With the improvement of living standards, it has become a basic requirement for textiles that have been treated with softeners to have good hydrophilicity. Therefore, the development of silicone softeners with better feel and hydrophilicity has become an urgent market demand.
Hydrophilic silicone softeners are generally side-chain polyether-modified silicone oil (commonly known as CGF in the market) and side-chain polyether-modified amino silicone oil. This kind of softener has good hydrophilicity, but the hand feeling of finished fabric is not ideal, which greatly limits its further application. The latest product structure is a ternary block type, and all functional functional groups are located in the main chain of silicone. The finished fabric not only has better hydrophilicity, but also has a relatively prominent hand feeling.
In this paper, four commonly used tertiary amines were reacted with epoxy-terminated silicone oils to prepare four hydrophilic block silicone oils, and their physical and chemical properties and application properties were compared.
2. Reaction principle [1-2]
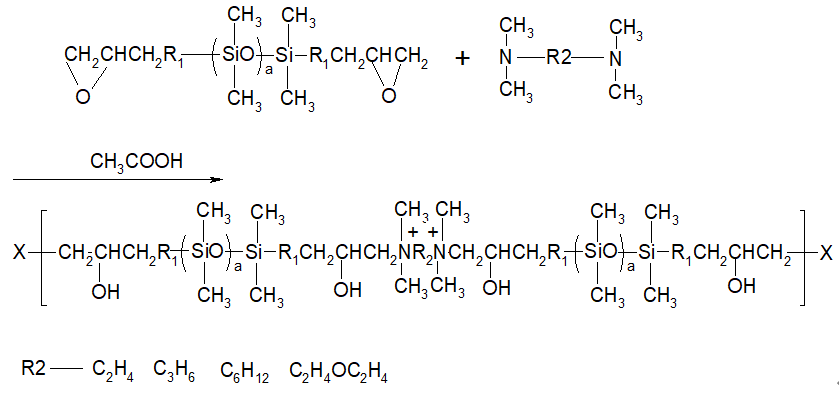
3. test
3.1 Materials and instruments
Fabrics: Whitening cotton knitted fabric; polyester-cotton knitted fabric (65/35)
Raw materials:
tetramethylethylenediamine, tetramethylpropylenediamine, tetramethylhexamethylenediamine, bis(dimethylaminoethyl) ether: commercially available;
epoxy-terminated silicone oil (M=8000g/mol): homemade;
Thirteen isomeric alcohol polyoxyethylene (TO5): commercially available;
isopropanol: analytically pure;
acetic acid: analytically pure.
Apparatus:
WSD whiteness meter, vacuum pump, Galaxy brand CS101-1AB electric heating drying oven.
3.2 Finishing process
One dipping and one rolling (75% pick up) → pre-baking (90°C, 2min) → baking (160°C, 2min)
3.3 Test [3-5]
3.3.1 Flexibility
Using the physical feel evaluation method, a professional team composed of 5 industry-literate personnel to evaluate the fabric feel. After passing the multi-person hand sensing evaluation, first, the ranking method is used to determine the fabric with the best hand feel and the worst fabric, and then the fabric with the best hand feel is rated as 5 points, and the worst fabric is rated as 1 point. The hand feeling of the other fabrics is obtained by the comparison method, which is divided into 1 to 5 grades, the larger the value, the softer.
3.3.2 Determination of whiteness
The spectral reflectance of the fabric finished with silicone softener was measured by fabric whiteness tester. Compared with the blank fabric, the larger the whiteness of the finished fabric is, that is, the whiteness difference with the blank fabric is smaller, indicates that the degree of color change of the fabric is smaller.
3.3.3 Hydrophilicity
The fabric finished with silicone softeners was laid flat on a horizontal plane, and drip 1 drop of water to fabric surface from 4 cm height with standard dropper ( 25 drops / ml ), then measure the time it takes for the fabric to absorb 1 drop of water under static conditions. The shorter the time, the better the hydrophilicity.
3.3.4 Washing fastness
Automatic testing machine of shrinkage rate, special detergent 1g/L, wash at 40℃ for 30min, wash 5 times, test the hand feel and hydrophilicity of the fabric after washing.
3.3.5 Centrifugal stability
Add 5 mL of emulsion to a 15 mL centrifuge test tube and put it in the centrifuge sedimenter; after rotating at a speed of 3000 r/min for 30 minutes, the sample does not delaminate and the quality is stable.
3.3.6 Stability of acid and alkali resistance
Put 5g emulsion, 95mL acetic acid solution (PH=2~3) or 95mL soda ash solution (PH=11~12) in the beaker, shake well; after standing for 24h, the sample has no delamination or demulsification phenomenon, which is of stable quality.
3.4 Synthesis of hydrophilic block modified silicone oil
In a 500mL three-necked flask equipped with a stirrer, condenser, and thermometer, add 0.4mol of epoxy-terminated silicone oil, 0.44mol of tertiary amine, a certain amount of acetic acid, and a certain amount of isopropanol as the solvent, and the temperature is increased to 82°C. The heat preservation reaction is 8 hours, the light yellow transparent viscous liquid is obtained, which is the hydrophilic block modified silicone oil.
3.5 Emulsification of hydrophilic block modified silicone oil
First add 10g tridecyl alcohol polyoxyethylene ether (TO7) into the beaker, then add 30g modified silicone oil, then add 0.6g glacial acetic acid, start the mixer, mix well, slowly add 59.4g deionized water, continue Stir continuously to obtain a blue transparent emulsion.
4. Results and discussion [6-11]
4.1 Stability of emulsion
The stability status of different organosilicon finishing agents is shown in Table 1.
The data in Table 1 are the various stability test data of 20g/L working fluid of various silicone oil emulsions with a solid content of 30%.
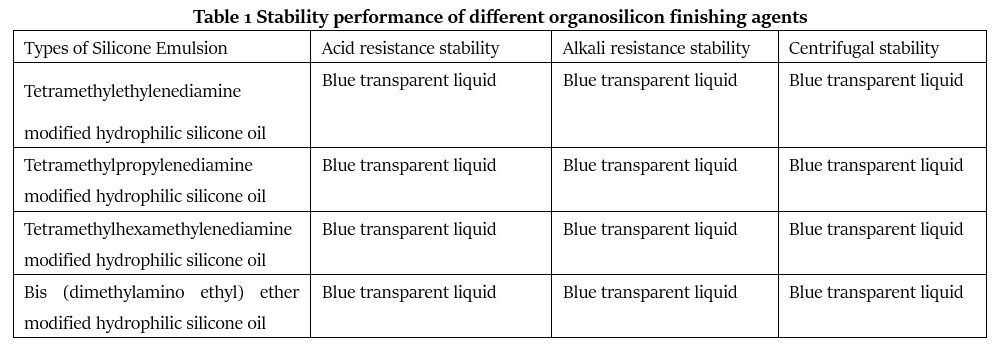
It can be seen from Table 1 that the four types of tertiary amine modified hydrophilic silicone oils all have high stability. The appearance of the emulsion under acid (PH=2~3), alkaline (PH=11~12) and centrifugal action No significant changes have occurred, and the original blue light transparent state is maintained. These hydrophilic silicone oil emulsions can maintain high stability under acidic and alkaline conditions, which are determined by their unique amino block modified structure uniformly distributed on the silicone backbone. The amino groups in the structure are acidic. Under conditions, it can form ammonium salt with acid to increase its polarity, so that it can have better solubility in water, and the cationic group in the block structure has better stability under alkaline conditions. Excellent acid and alkali resistance, so that it has a wider range of applications.
4.2 Whiteness
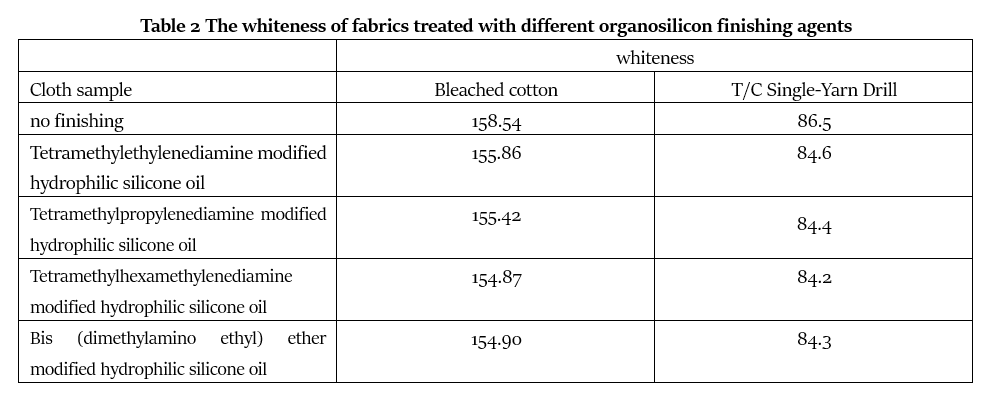
It can be seen from Table 2 that the whiteness of the finished fabric is lower than that of the unfinished blank fabric to a certain extent. The four tertiary amine-modified hydrophilic silicone oils have similar effects on the whiteness of the fabric. This is because their structures are relatively similar. They are all modified with the main chain quaternary ammonium salt block structure and have strong antioxidant capacity. The finished fabric has strong resistance to yellowing, and the whiteness decline is small. Tetramethylethylenediamine modified hydrophilic silicone oil and tetramethylpropylenediamine modified hydrophilic silicone oil have slightly less influence on the whiteness of fabrics. Tetramethylethylenediamine modified hydrophilic silicone oil and bis(dimethylamino) Ethyl ether modified hydrophilic silicone oil has a slightly greater impact on the whiteness of fabrics.
4.3 Hydrophilicity
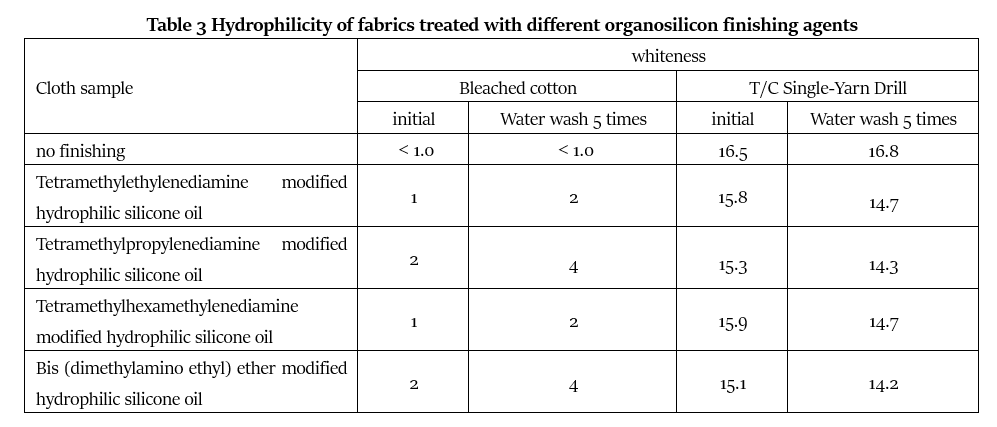
It can be seen from Table 3 that the hydrophilicity of bleached cotton fabrics and polyester-cotton yarns after treatment with silicone finishing agents is reduced to varying degrees compared with untreated fabrics. Among them, the tetramethylethylenediamine modified hydrophilic silicone oil and the tetramethylhexamethylenediamine modified hydrophilic silicone oil have slightly less influence on the hydrophilicity of the fabric, and the tetramethylethylenediamine modified hydrophilic silicone oil has a little (Methylaminoethyl) ether modified hydrophilic silicone oil has a slightly greater impact on the hydrophilicity of the fabric, but the degree of impact is roughly the same, and the hydrophilicity is faster. This is related to the large number of cationic quaternary ammonium salt groups in its structure. This group has very excellent hydrophilic properties, and at the same time its ability to bind to fibers is also strong, so it has good washing resistance.
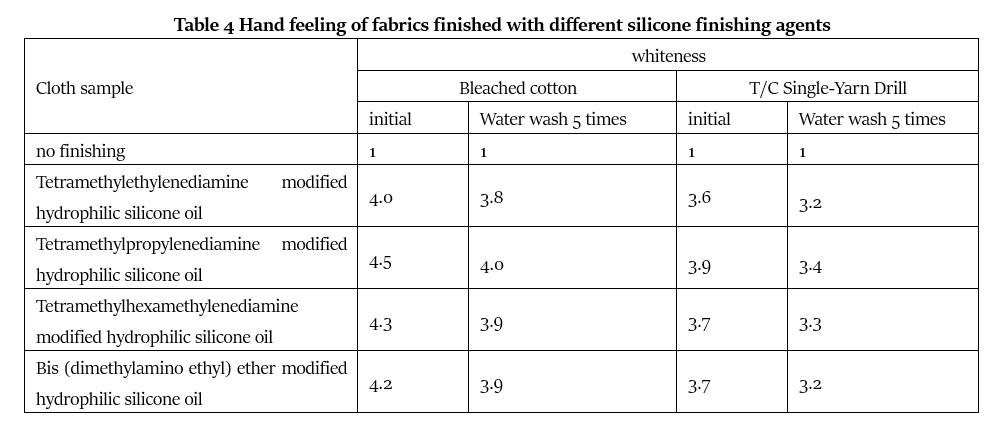
4.4 Hand feeling
It can be seen from the data in Table 4 that the hand feel of the fabrics treated with different finishing agents has been improved to varying degrees. Among them, the hand feel of the fabrics after finishing with tetramethylethylenediamine modified hydrophilic silicone oil is slightly worse. The hexamethylene diamine modified hydrophilic silicone oil, the tetramethyl propylene diamine modified hydrophilic silicone oil and the bis (dimethylamino ethyl) ether modified hydrophilic silicone oil have the same hand feeling to the fabric after finishing, and the washing performance is better. When the silicone softener is attached to the fabric, it will form a macromolecular film with a linear or net-like structure between the fibers and on the surface of the fabric, thereby avoiding direct contact between the fiber and the fiber, and between the yarn and the fabric. Contact effectively reduces the friction coefficient of the yarns in the fabric, so that the fibers can slide relatively under a small external force to produce a smooth effect, and the fabric is easy to deform and produce a soft feel. These four tertiary amine modified hydrophilic silicone oils all contain highly polar groups, which can interact with the ester groups, carboxyl groups, hydroxyl groups, amide groups, etc. in the fiber to form strong directional adsorption and orientation, so that the finishing The resulting fabric has a good hand feel and excellent washability.
In addition, these four tertiary amines modified hydrophilic silicone oils show different washability and hand feeling on bleached cotton cloth and polyester-cotton yarn. The main reason may be that the finishing agent contains a large amount of amidated or quaternized molecules. Nitrogen atoms are related, and their interaction with fibers is mainly van der Waals force. The polyester fiber in the polyester-cotton yarn card and the nitrogen atom of the structure has a large dimensional resistance, and the interaction force is weak. The finishing agent of the structure has a small adsorption capacity; while the bleached cotton cloth contains a large number of polar groups, the quaternized nitrogen atoms in the molecules of these four tertiary amines modified hydrophilic silicone oils can form a strong chemical bond with them to form a thread The macromolecular film of the shape or net shape, so it has a good hand feeling and washing resistance on bleached cotton cloth, but it is poor on T/C Single-Yarn Drill.
5. Conclusion
(1) Four hydrophilic block silicone oils were obtained by reacting four common tertiary amines, tetramethylethylenediamine, tetramethylpropanediamine, tetramethylhexanediamine and bis ( dimethylaminoethyl ) ether, with epoxy-terminated silicone oil. These four hydrophilic softeners have excellent acid resistance, alkali resistance and centrifugal stability.
(2) The results show that the hydrophilic block silicone oil synthesized by three tertiary amines, tetramethylpropanediamine, tetramethylhexanediamine and bis (dimethylaminoethyl ) ether, has excellent whiteness, hydrophilicity, handle and washing resistance on bleached cotton cloth and T/C Single-Yarn Drill.
(3) In the synthesis of hydrophilic block silicone oil, the tertiary amines of tetramethylpropanediamine, tetramethylhexanediamine and bis ( dimethylaminoethyl ) ether have high mutual substitution.
References :
[1] Huang Wenrun. Silicone oil and its application [M]. Sichuan: Sichuan Science and Technology Press, 2018.150-175.
[2] WALTER NOLL. Chemistry and technology of silicones [M]. Academic Press, New York, 1968:303.
[3] Hu Canhui, Zhu Quan, Guo Yuliang. Application performance of hydrophilic silicone softener [J]. Printing and Dyeing, 2016 (7): 31-33.
[4] Liu Ruiyun. Synthesis and application of main chain polyether block modified silicone oil BW [C]. Proceedings of the Fifth (Guangdong) Textile Auxiliary Industry Annual Conference, 2013: 129-133.
[5] Li Huanling, Zheng Xiaoshan, Li Li. Wetting performance of trisiloxane surfactants on low-energy surfaces [J]. Silicone Materials, 2019, 33 (2): 91-96.
[6]Block, Non-(AB)n Silicone polyalkyleneoxide Copolymers With Tertiary Amino Links: United States, 6475568B1[P].2002-11-05
[7] Liu Ruiyun. Synthesis and application of main chain block hydrophilic amino silicone oil [J]. Printing and Dyeing Auxiliaries, 2011 (5): 48-50.
[8]ANGELO J S.Modification of the tactile and physical properties of microfiber fabric blends with silicone polymers [J].Text Chem Color,1995,27(9):79-81.
[9] Liu Ruiyun, Miao Yaosheng, Jiang Yingmei. Synthesis and Application of Polyether Block Modified Silicone Oil [J]. Organosilicon Materials, 2012, 26(6): 396-399.
[10]Sun Fuqian, Zhang Peng, Wang Xiaoyu. Study on the synthesis and properties of quaternary ammonium salt type silicone oil[J]. Materials Guide, 2017 (5): 342-345.
[11] Huang Wenrun. Amino-modified polyorganosiloxane and then modified soft finishing agent [J]. Organosilicon materials, 2001 Supplement: 40-56.
BIOGRAPHIES
Liu Ruiyun
Master of textile engineering, Liu graduated from Dyeing and Finishing Engineering, Department of Chemistry and Chemical Engineering, Northwest Institute of Textile Technology (now Xi'an University of Technology). He successively worked in Zhangjiagang Guotai Huarong and Shanghai Argus Textile Auxiliaries company. In 2021, Mr. Liu joined OSiC textile department and mainly engaged in the development and research of silicone auxiliaries and textile chemicals.
OSiC Performance Materials
Textile Auxiliaries
No.1218, Songsheng Road
201600 Shanghai
China
Abstract: Four kinds of hydrophilic block silicone oil were obtained by reaction of tetramethylethylenediamine, tetramethyl propylenediamine, tetramethylhexanediamine and bis ( dimethylaminoethyl ) ether with epoxy silicone oil. The physicochemical properties of these four hydrophilic softeners, such as acid resistance, alkali resistance, anion resistance and shear resistance, were investigated and their application properties were studied. The results show that the hydrophilic block silicone oil synthesized by using three tertiary amines of tetramethylpropylenediamine, tetramethylhexamethylenediamine and bis(dimethylaminoethyl)ether has the same handle and hydrophilic properties.
Key words: Tertiary amine; hydrophilic; block silicone oil; properties
1. Introduction
Textiles treated with conventional silicone softeners generally suffer from poor hydrophilicity, hot stuffy wearing, and reduced comfort. With the improvement of living standards, it has become a basic requirement for textiles that have been treated with softeners to have good hydrophilicity. Therefore, the development of silicone softeners with better feel and hydrophilicity has become an urgent market demand.
Hydrophilic silicone softeners are generally side-chain polyether-modified silicone oil (commonly known as CGF in the market) and side-chain polyether-modified amino silicone oil. This kind of softener has good hydrophilicity, but the hand feeling of finished fabric is not ideal, which greatly limits its further application. The latest product structure is a ternary block type, and all functional functional groups are located in the main chain of silicone. The finished fabric not only has better hydrophilicity, but also has a relatively prominent hand feeling.
In this paper, four commonly used tertiary amines were reacted with epoxy-terminated silicone oils to prepare four hydrophilic block silicone oils, and their physical and chemical properties and application properties were compared.
2. Reaction principle [1-2]

3. test
3.1 Materials and instruments
Fabrics: Whitening cotton knitted fabric; polyester-cotton knitted fabric (65/35)
Raw materials:
tetramethylethylenediamine, tetramethylpropylenediamine, tetramethylhexamethylenediamine, bis(dimethylaminoethyl) ether: commercially available;
epoxy-terminated silicone oil (M=8000g/mol): homemade;
Thirteen isomeric alcohol polyoxyethylene (TO5): commercially available;
isopropanol: analytically pure;
acetic acid: analytically pure.
Apparatus:
WSD whiteness meter, vacuum pump, Galaxy brand CS101-1AB electric heating drying oven.
3.2 Finishing process
One dipping and one rolling (75% pick up) → pre-baking (90°C, 2min) → baking (160°C, 2min)
3.3 Test [3-5]
3.3.1 Flexibility
Using the physical feel evaluation method, a professional team composed of 5 industry-literate personnel to evaluate the fabric feel. After passing the multi-person hand sensing evaluation, first, the ranking method is used to determine the fabric with the best hand feel and the worst fabric, and then the fabric with the best hand feel is rated as 5 points, and the worst fabric is rated as 1 point. The hand feeling of the other fabrics is obtained by the comparison method, which is divided into 1 to 5 grades, the larger the value, the softer.
3.3.2 Determination of whiteness
The spectral reflectance of the fabric finished with silicone softener was measured by fabric whiteness tester. Compared with the blank fabric, the larger the whiteness of the finished fabric is, that is, the whiteness difference with the blank fabric is smaller, indicates that the degree of color change of the fabric is smaller.
3.3.3 Hydrophilicity
The fabric finished with silicone softeners was laid flat on a horizontal plane, and drip 1 drop of water to fabric surface from 4 cm height with standard dropper ( 25 drops / ml ), then measure the time it takes for the fabric to absorb 1 drop of water under static conditions. The shorter the time, the better the hydrophilicity.
3.3.4 Washing fastness
Automatic testing machine of shrinkage rate, special detergent 1g/L, wash at 40℃ for 30min, wash 5 times, test the hand feel and hydrophilicity of the fabric after washing.
3.3.5 Centrifugal stability
Add 5 mL of emulsion to a 15 mL centrifuge test tube and put it in the centrifuge sedimenter; after rotating at a speed of 3000 r/min for 30 minutes, the sample does not delaminate and the quality is stable.
3.3.6 Stability of acid and alkali resistance
Put 5g emulsion, 95mL acetic acid solution (PH=2~3) or 95mL soda ash solution (PH=11~12) in the beaker, shake well; after standing for 24h, the sample has no delamination or demulsification phenomenon, which is of stable quality.
3.4 Synthesis of hydrophilic block modified silicone oil
In a 500mL three-necked flask equipped with a stirrer, condenser, and thermometer, add 0.4mol of epoxy-terminated silicone oil, 0.44mol of tertiary amine, a certain amount of acetic acid, and a certain amount of isopropanol as the solvent, and the temperature is increased to 82°C. The heat preservation reaction is 8 hours, the light yellow transparent viscous liquid is obtained, which is the hydrophilic block modified silicone oil.
3.5 Emulsification of hydrophilic block modified silicone oil
First add 10g tridecyl alcohol polyoxyethylene ether (TO7) into the beaker, then add 30g modified silicone oil, then add 0.6g glacial acetic acid, start the mixer, mix well, slowly add 59.4g deionized water, continue Stir continuously to obtain a blue transparent emulsion.
4. Results and discussion [6-11]
4.1 Stability of emulsion
The stability status of different organosilicon finishing agents is shown in Table 1.
The data in Table 1 are the various stability test data of 20g/L working fluid of various silicone oil emulsions with a solid content of 30%.

It can be seen from Table 1 that the four types of tertiary amine modified hydrophilic silicone oils all have high stability. The appearance of the emulsion under acid (PH=2~3), alkaline (PH=11~12) and centrifugal action No significant changes have occurred, and the original blue light transparent state is maintained. These hydrophilic silicone oil emulsions can maintain high stability under acidic and alkaline conditions, which are determined by their unique amino block modified structure uniformly distributed on the silicone backbone. The amino groups in the structure are acidic. Under conditions, it can form ammonium salt with acid to increase its polarity, so that it can have better solubility in water, and the cationic group in the block structure has better stability under alkaline conditions. Excellent acid and alkali resistance, so that it has a wider range of applications.
4.2 Whiteness
It can be seen from Table 2 that the whiteness of the finished fabric is lower than that of the unfinished blank fabric to a certain extent. The four tertiary amine-modified hydrophilic silicone oils have similar effects on the whiteness of the fabric. This is because their structures are relatively similar. They are all modified with the main chain quaternary ammonium salt block structure and have strong antioxidant capacity. The finished fabric has strong resistance to yellowing, and the whiteness decline is small. Tetramethylethylenediamine modified hydrophilic silicone oil and tetramethylpropylenediamine modified hydrophilic silicone oil have slightly less influence on the whiteness of fabrics. Tetramethylethylenediamine modified hydrophilic silicone oil and bis(dimethylamino) Ethyl ether modified hydrophilic silicone oil has a slightly greater impact on the whiteness of fabrics.

4.3 Hydrophilicity
It can be seen from Table 3 that the hydrophilicity of bleached cotton fabrics and polyester-cotton yarns after treatment with silicone finishing agents is reduced to varying degrees compared with untreated fabrics. Among them, the tetramethylethylenediamine modified hydrophilic silicone oil and the tetramethylhexamethylenediamine modified hydrophilic silicone oil have slightly less influence on the hydrophilicity of the fabric, and the tetramethylethylenediamine modified hydrophilic silicone oil has a little (Methylaminoethyl) ether modified hydrophilic silicone oil has a slightly greater impact on the hydrophilicity of the fabric, but the degree of impact is roughly the same, and the hydrophilicity is faster. This is related to the large number of cationic quaternary ammonium salt groups in its structure. This group has very excellent hydrophilic properties, and at the same time its ability to bind to fibers is also strong, so it has good washing resistance.

4.4 Hand feeling
It can be seen from the data in Table 4 that the hand feel of the fabrics treated with different finishing agents has been improved to varying degrees. Among them, the hand feel of the fabrics after finishing with tetramethylethylenediamine modified hydrophilic silicone oil is slightly worse. The hexamethylene diamine modified hydrophilic silicone oil, the tetramethyl propylene diamine modified hydrophilic silicone oil and the bis (dimethylamino ethyl) ether modified hydrophilic silicone oil have the same hand feeling to the fabric after finishing, and the washing performance is better. When the silicone softener is attached to the fabric, it will form a macromolecular film with a linear or net-like structure between the fibers and on the surface of the fabric, thereby avoiding direct contact between the fiber and the fiber, and between the yarn and the fabric. Contact effectively reduces the friction coefficient of the yarns in the fabric, so that the fibers can slide relatively under a small external force to produce a smooth effect, and the fabric is easy to deform and produce a soft feel. These four tertiary amine modified hydrophilic silicone oils all contain highly polar groups, which can interact with the ester groups, carboxyl groups, hydroxyl groups, amide groups, etc. in the fiber to form strong directional adsorption and orientation, so that the finishing The resulting fabric has a good hand feel and excellent washability.
In addition, these four tertiary amines modified hydrophilic silicone oils show different washability and hand feeling on bleached cotton cloth and polyester-cotton yarn. The main reason may be that the finishing agent contains a large amount of amidated or quaternized molecules. Nitrogen atoms are related, and their interaction with fibers is mainly van der Waals force. The polyester fiber in the polyester-cotton yarn card and the nitrogen atom of the structure has a large dimensional resistance, and the interaction force is weak. The finishing agent of the structure has a small adsorption capacity; while the bleached cotton cloth contains a large number of polar groups, the quaternized nitrogen atoms in the molecules of these four tertiary amines modified hydrophilic silicone oils can form a strong chemical bond with them to form a thread The macromolecular film of the shape or net shape, so it has a good hand feeling and washing resistance on bleached cotton cloth, but it is poor on T/C Single-Yarn Drill.

5. Conclusion
(1) Four hydrophilic block silicone oils were obtained by reacting four common tertiary amines, tetramethylethylenediamine, tetramethylpropanediamine, tetramethylhexanediamine and bis ( dimethylaminoethyl ) ether, with epoxy-terminated silicone oil. These four hydrophilic softeners have excellent acid resistance, alkali resistance and centrifugal stability.
(2) The results show that the hydrophilic block silicone oil synthesized by three tertiary amines, tetramethylpropanediamine, tetramethylhexanediamine and bis (dimethylaminoethyl ) ether, has excellent whiteness, hydrophilicity, handle and washing resistance on bleached cotton cloth and T/C Single-Yarn Drill.
(3) In the synthesis of hydrophilic block silicone oil, the tertiary amines of tetramethylpropanediamine, tetramethylhexanediamine and bis ( dimethylaminoethyl ) ether have high mutual substitution.
References :
[1] Huang Wenrun. Silicone oil and its application [M]. Sichuan: Sichuan Science and Technology Press, 2018.150-175.
[2] WALTER NOLL. Chemistry and technology of silicones [M]. Academic Press, New York, 1968:303.
[3] Hu Canhui, Zhu Quan, Guo Yuliang. Application performance of hydrophilic silicone softener [J]. Printing and Dyeing, 2016 (7): 31-33.
[4] Liu Ruiyun. Synthesis and application of main chain polyether block modified silicone oil BW [C]. Proceedings of the Fifth (Guangdong) Textile Auxiliary Industry Annual Conference, 2013: 129-133.
[5] Li Huanling, Zheng Xiaoshan, Li Li. Wetting performance of trisiloxane surfactants on low-energy surfaces [J]. Silicone Materials, 2019, 33 (2): 91-96.
[6]Block, Non-(AB)n Silicone polyalkyleneoxide Copolymers With Tertiary Amino Links: United States, 6475568B1[P].2002-11-05
[7] Liu Ruiyun. Synthesis and application of main chain block hydrophilic amino silicone oil [J]. Printing and Dyeing Auxiliaries, 2011 (5): 48-50.
[8]ANGELO J S.Modification of the tactile and physical properties of microfiber fabric blends with silicone polymers [J].Text Chem Color,1995,27(9):79-81.
[9] Liu Ruiyun, Miao Yaosheng, Jiang Yingmei. Synthesis and Application of Polyether Block Modified Silicone Oil [J]. Organosilicon Materials, 2012, 26(6): 396-399.
[10]Sun Fuqian, Zhang Peng, Wang Xiaoyu. Study on the synthesis and properties of quaternary ammonium salt type silicone oil[J]. Materials Guide, 2017 (5): 342-345.
[11] Huang Wenrun. Amino-modified polyorganosiloxane and then modified soft finishing agent [J]. Organosilicon materials, 2001 Supplement: 40-56.
BIOGRAPHIES
Liu Ruiyun
Master of textile engineering, Liu graduated from Dyeing and Finishing Engineering, Department of Chemistry and Chemical Engineering, Northwest Institute of Textile Technology (now Xi'an University of Technology). He successively worked in Zhangjiagang Guotai Huarong and Shanghai Argus Textile Auxiliaries company. In 2021, Mr. Liu joined OSiC textile department and mainly engaged in the development and research of silicone auxiliaries and textile chemicals.